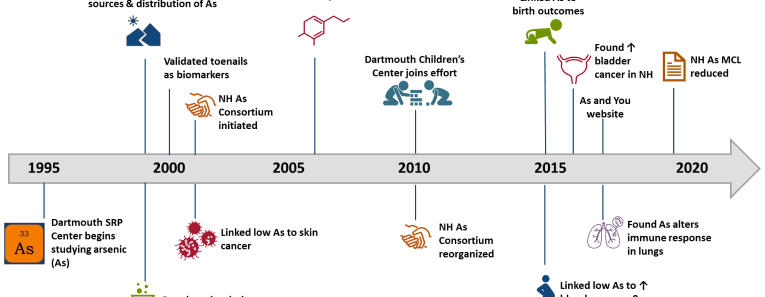
The Dartmouth Toxic Metals Superfund Research Program uses an interdisciplinary approach to investigate the ways in which arsenic and mercury in the environment affect ecosystems and human health. We communicate our results to communities, grass-roots organizations, and state and federal agencies, and we train students to conduct research from both a clinical and community-based perspective. We hope you will be inspired to ask questions about our work, and will learn about the ways these metals may affect your health.