Dartmouth College Institutional Biosafety Committee
What is the Institutional Biosafety Committee (IBC)?
The Institutional Biological Safety Committee (IBC) provides review and oversight of the College’s Biosafety Program in accordance with all local, state, and federal regulations and guidelines. The Dartmouth IBC reviews all biological research on campus to ensure labs have proper containment for various types of biological agents, including recombinant DNA, infectious agents, toxins, human source materials, etc., and that all PIs and lab personnel are properly trained in biosafety. As such, the IBC serves as an advisory board to the research community on all aspects of biological safety.
Do I need to submit my research for IBC review?
Yes. All biological research at Dartmouth needs IBC review and approval before commencement.
Why do I have to submit my work to the IBC?
The NIH requires all institutions receiving research funds to have its research reviewed by an IBC (regardless of whether that research is directly supported by the NIH), as stipulated by the NIH Guidelines for Research Involving Recombinant or Synthetic DNA Molecules. If research is conducted without approval, the NIH has the authority to withdrawal research support from that lab or institution.
Section IV-B-7 of the NIH Guidelines states, "on behalf of the institution, the Principal Investigator is responsible for full compliance with the NIH Guidelines". For more information on PI Responsibilities under the NIH Guidelines, please refer to our PI Responsibilities webpage.
What is the IBC Review Process?
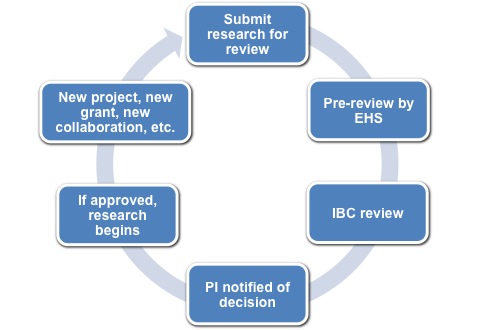
The research review process by the IBC is conducted in the step-wise fashion above, starting with the submission of research information by the PI.
1. Submit your research to the IBC: Online submission to the IBC is achieved using the BioRAFT online registration system (see below for submission instructions. Click here for BioRAFT Basics). All information pertaining to biological agent usage (rDNA technology - including viral vectors, animal research, human source material, infectious agents, biotoxins, etc.) and the personnel involved with the research will be queried in this system. This information is maintained in BioRAFT and you can update or revise it any time there is a change in your research program.
2. EHS Pre-Review The Biological Safety Officer will review your research information and conduct a risk assessment based upon the information submitted. A biosafety audit of the lab will be conducted as part of the risk assessment. Any questions/problems/concerns will be directed back to the PI for clarification. Once all aspects of the risk assessment have been satisfied by EHS, your research will be reviewed by the IBC committee.
3. IBC Review The IBC will review the research information submitted through BioRAFT and discuss the safety and regulatory aspects of the research project. Discussion topics include: the nature of experimentation, location, rDNA aspects, training requirements fulfilled by research staff, past laboratory inspection record, etc. If it is warranted, researchers can be invited to present their research protocols to the IBC committee.
4. Decision Letter Sent to PI Once the IBC has made a decision regarding the research project reviewed, a decision letter will be sent to the PI from the Biological Safety Officer and the Chair of the IBC. This decision letter should be retained for your records.
5. Conduct Research Once an approval letter has been received from the IBC, only then can the proposed research begin.
6. Calendar with IBC committee meeting and submission deadlines is available here.
How do I submit my research for IBC review?
At Dartmouth, we use BioRAFT as the online application mechanism for IBC approval. To submit your research to the IBC, you need to enter all relevant information into the BioRAFT "Bio Summary" (aka "Usage Summary" or "Bio Registration" or "IBC Registration"). The BioRAFT Bio Summary is designed to communicate the biohazardous aspects of your research to the IBC so that it can properly review and approve your work. The registration of your lab in BioRAFT is conveniently setup to ask you specific questions ("surveys") about your research to make this process easier. As the PI, you will be required to complete a series of surveys in BioRAFT that are aimed to identify the biohazards involved in your research program (rDNA, cells, animals, toxins, human source materials/cells, pathogenic microbes, viruses, etc). If you have already have a Bio Summary and need to update it, you can edit any of these surveys to make the necessary modifications. This allows you to have a "live" snapshot of current research in your lab - how nice!
Once this information has been inputted, BioRAFT will then prompt you to "certify" that your Bio Summary (registration) is complete and correct. Once certified, you will be asked to initial several compliance statements before submitting the registration. IMPORTANT NOTE: The information will only be sent to the Biological Safety Officer for pre-review after it has been certified and submitted by the PI.
The Biological Safety Officer (BSO) will then review your BioRAFT Bio Summary, discuss any questions or concerns with you during a risk assessment (which may include a lab safety audit). The BSO then creates a Summary Report of your submitted research and presents it to the IBC on your behalf. Thus, it is in your best interest that the information you provide in BioRAFT is complete and accurate so that the BSO can accurately present your research to the IBC. Lacking or incorrect information may delay your approval process. Any PI is welcome to present his/her work directly to the IBC if he/she would like.
Notes:
-
To obtain IBC approval without contingencies, all lab members must be up-to-date on mandatory Dartmouth College laboratory safety training. All training is provided online in BioRAFT, and you can quickly check your lab's compliance in the Compliance Summary box of your lab's main page in BioRAFT.
-
IBC approval is good for 3 years; however, any new research (new funding, new collaborations, etc.) is reviewed ad hoc as a modification.
-
If you would like help completing your BioRAFT Biological Registration, please contact Erik Pietrowicz for assistance.
IBC Charter
The Dartmouth IBC Charter covers all of the working procedures for the review of biological research and oversight authorities of the committee.
The IBC meets every other month, or more frequently as needed, and meetings are open to the public.
IBC for Clinical Gene Transfer (IBC-CGT)
The Dartmouth IBC for Clinical Gene Transfer (IBC-CGT) is a separate IBC charged with the review of human gene transfer experiments. Please click here for info on how to submit a human gene transfer protocol to the IBC-CGT.
IBC Members:
Joshua Obar, PhD, IBC Chair, Associate Professor, Microbiology and Immunology
Mary Kay Brown, MS, Instructor and Lab Manager, Thayer Engineering
Renee Brown, Senior Grants Associate, Office of Sponsored Projects
Frank Gesek, PhD, VA Representative
Matt Hayden, MD, PhD, Assistant Professor of Dermatology
Thomas Jack, PhD, Professor, Biological Sciences
Kimberly Lyford, Senior Human Research Analyst, Committee for the Protection of Human Subjects
Kirk J. Maurer, DVM, PhD, Attending Veterinarian, Center for Comparative Medicine and Research
Susan McCoy, MHS, Community Representative
Erik Pietrowicz, MS, Biological Safety Officer, Environmental Health & Safety
Benjamin Ross, PhD, Assistant Professor, Microbiology and Immunology
Terryl Stacy, PhD, Community Representative



